Design takes into account process and personnel flow cross-contamination. The system will also be placed under formal change control in.
How To Create A Cgmp Compliant Installation Qualification Protocol
The cleanroom validation protocol may include the following 14 challenges and testing activities.

. All of the tests were performed and a report was generated. This protocol will be executed in compliance as per the requirements in 21CFR 210 211 ICH Q-7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients August 2001. Design meets the user requirements.
Document Location of the latest versions. QUALIFICATION OF DYNAMIC PASS BOXDPB HVAC QUALIFICATION. Design Qualification Protocol HVAC.
The cleanroom validation must be performed after completed the facility and HVAC qualification of all equipment installation and machinery connections supplies air conditioning water system compresses air system electric power capacity. This combined Facility Utility HVAC qualification validation SOP and IQ template makes it so easy for you to raise a quality IQ. Design qualification is defined as a verification process on the design to meet particular requirements relating to the quality of pharmaceuticals and manufacturing practices.
Design details facility airflow and pressure cascade philosophy. This qualification protocol document package pertains specifically to the Taylor-Wharton Stainless Steel Cryogenic Freezer which serve as a product storage container and com-prised of. Connections are in compliance with the design qualification and also with as-built drawing.
We offer the right solutions for an improved and clean environment in working and production areas and. This protocol will be performed utilizing 21 CFR 210 211 ICH Q-7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients August 2001. Template for Design Qualification Protocol OBJECTIVE To design engineer and supply the Name of Equipment and to provide assurance that the machine is manufactured as per the URS.
However the procedure for design qualification in pharmaceuticals is one reason as to why some products do not make it to the shelves in drugstores. Finally the HVAC system was subjected to a performance qualification PQ study. Physically check the dimension of the AHU in length width and height and confirm with design document.
As built drawing 2. Viable particle count test. Approved Design Qualification Document TEST METHOD 1.
Aug 31 2015 Messages. Terminal and AHU-mounted HEPA filters Grade details leak tested Mechanical design mark-ups and updates carried out and ensure all components installed as shown Building Management System BMS configuration. VackerGlobal is one of the reputed specialists in HVAC validation qualification.
_____ pertaining to design specification of HVAC System AHU-06 2. Air Handling Unit Terminal Filters Diffusers. Following test shall be performed for DPB with the same Procedure as defined in HVAC qualification.
Upon final approval of this IQ Protocol and Summary Report it will replace the previous IQ study and render it obsolete. DQ Design Qualification PURPOSE. Become a Bronze Member and get products for free.
Narya Wijaya New Member. DQ is to verify that the system has been designed as specified in the URS User Requirements Specification FDS Functional Design Specification and relevant equipment specifications satisfying all GMP requirements. The final product is a professional and comprehensive HVAC Qualification Protocol.
B The premises supporting utilities and equipment have. Verify the accuracy of those drawings which represent the system as built. Discussion in Pharmaceuticals 21 CFR Parts 210 211 started by Narya Wijaya Sep 1 2015.
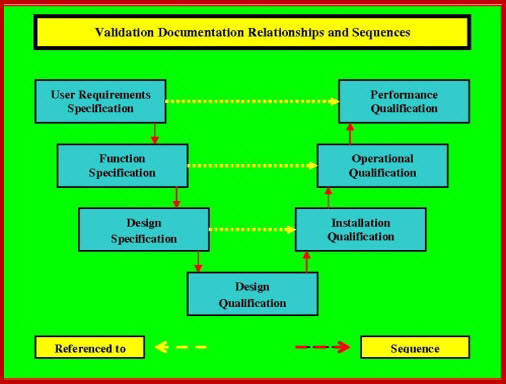
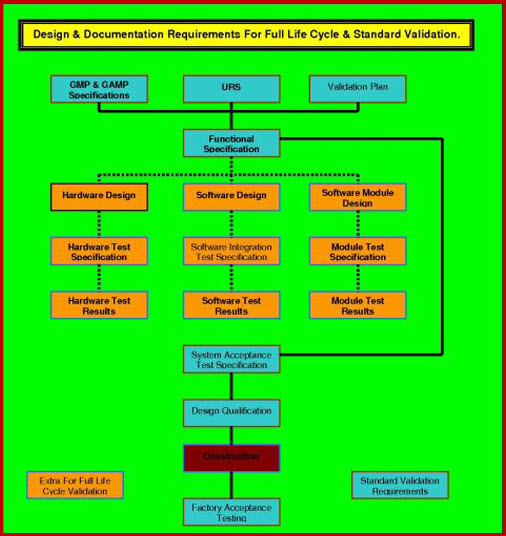
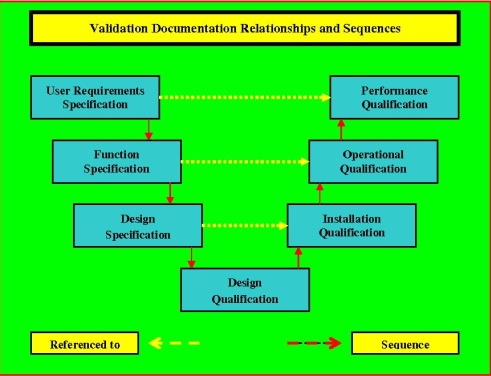
The HVAC validation comprises of four core aspects Design Qualification DQ Installation Qualification IQ Operational Qualification OQ and Performance Qualification PQ. On evaluation of the data collected during PQ it was found that the HVAC system met all the specified design criteria and complied with the entire cGMP requirement. A Design Qualification protocol is used at the stage where a design that has been developed from the VMP URS GAMP 5 cGMP and other Health and Safety Guidelines is reviewed and documented by competent persons to ensure that the designed equipment if built will satisfy all the detailed specified requirements as contained in the VP and URS.
Doc Number and it complies with the Scope of Supply. The Process User Requirements in the URS have been identified by the Quality Risk Assessment. With the new suite of HVAC Qualification documents from the DQ - IQ - OQ - PQ all user friendly and ready to go your validation life has got much simpler.
Equipment and processes have been designed in accordance with the requirements for GMP Design Qualification. DESIGN QUALIFICATION DQ 1. Installation Qualification IQ-HVAC Ensure that critical HVAC components are correctly installed.
To Re-qualify the HVAC system of All area and establish documentary evidence to demonstrate that Air Handling Units Ventilation Units Exhaust units Laminar Air Flow and Reverse Laminar Air Flow units are qualified to perform well within the predetermined acceptance limit of performance as per guidelines outlined in this protocol. To prove that each operation proceeds as per the design qualification and the tolerances prescribed there in. OBJECTIVE The objective of this document is to comply with URS Document Number.
SCOPE Design Specifications of HVAC System Comprising of the following main Equipments. Qualification should be performed for new premises equipment utilities and systems at periodic intervals when major changes have been made. A QUALIFICATION APPROACH FOR HVAC SYSTEMS A thoroughly executed DQ process ensures the following.
HVAC ELECTRICAL INSTALLATION Complete a list of drawings manuals associated with the electrical installation of the HVAC system. Hi all its good to have this forum that we can share each other since cove off. Compliance with GMPs and other regulatory requirements.
Vacuum Insulated Product Storage Container Control System This document package is intended to serve as a guide during the Design Qualification. Non-viable particle count test.
Hvac Operation Qualification Protocol

Design Qualification Dq Of Equipment Pharmaceutical Guidelines
Design Qualification Fda Mhra Ema Who Validation Online

Dq Iq Oq Pq Cleanroom Qualifications
Hvac Qualification Mhra Fda Eu Who Cgmp Sop Gamp 5
0 comments
Post a Comment